Useful Information
For international users, please contact Dr. Guan or Prof. Wang for detailed instructions.
Research interests of BL09U
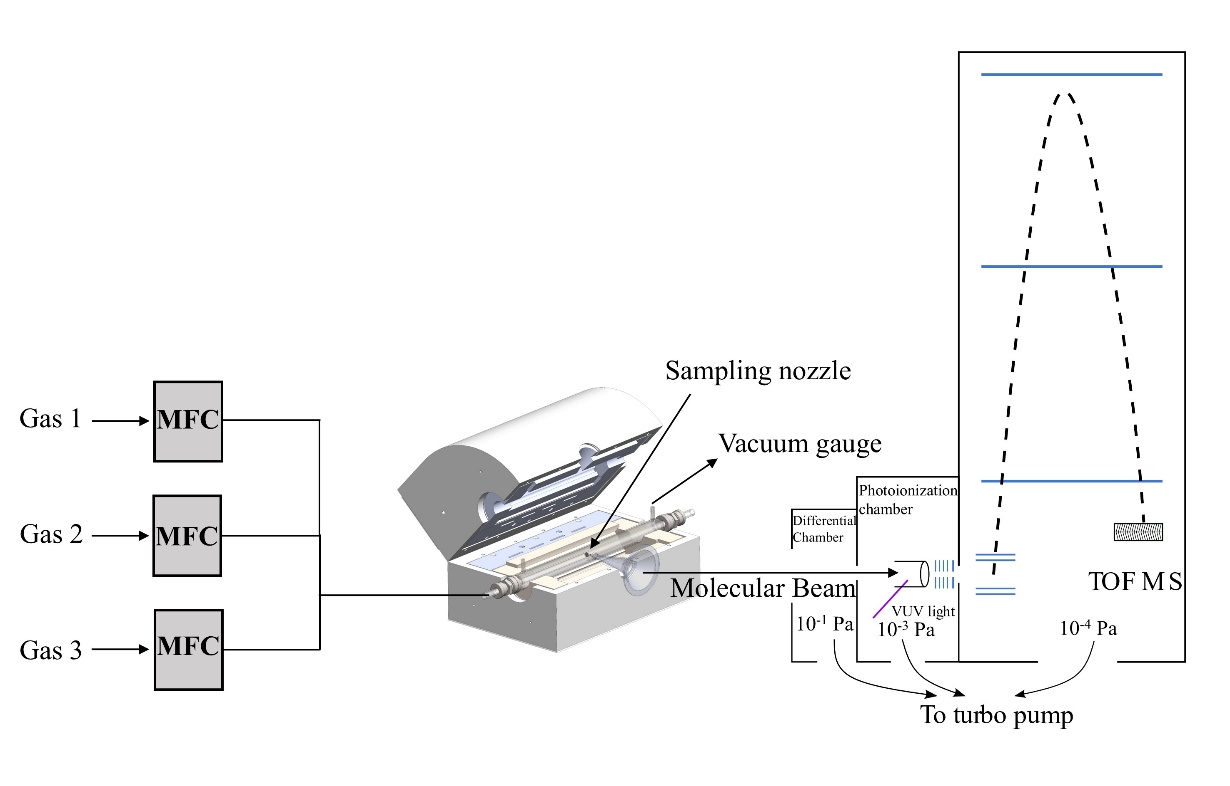
Principle of Synchrotron VUV Photoionization Mass Spectrometry
Synchrotron radiation photoionization mass spectrometry uses synchrotron vacuum ultraviolet light as the ionization source and combines it with time-of-flight mass spectrometry to detect the gaseous molecules. Synchrotron photoionization is a single-photon ionization technique that can achieve soft ionization of molecules. Compared with conventional 70 eV ionization of electron gun, synchrotron VUV single-photon ionization technology has less fragmentation and can achieve threshold photoionization of molecules. The tunable VUV photon energy covers almost all the ionization energies of organic matter. At the same time, due to the difference in ionization energies of isomeric substances, synchrotron VUV photoionization mass spectrometry can better distinguish isomeric substances. Combined with molecular beam sampling technology, molecular beam synchrotron radiation photoionization mass spectrometry is the most important means of online detection of intermediate species in complex reaction systems.
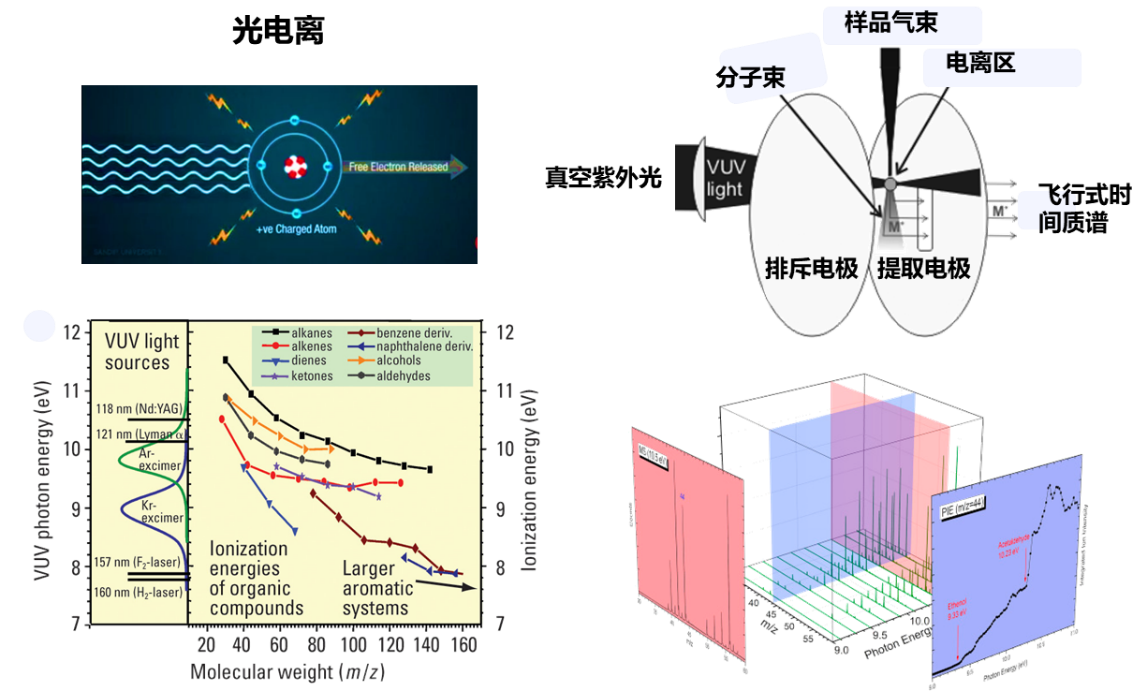
Applications
Aerospace propellant combustion mechanism
The study of aerospace propellant combustion is a core topic for improving engine performance and reliability. As spacecraft develop towards higher specific impulse and wider operating range, traditional propellants face challenges in combustion efficiency, stability and pollutant control. The generation and evolution of transient free radicals and intermediates in the combustion process directly affect the flame propagation speed, combustion oscillation characteristics and carbon deposition formation mechanism, but traditional detection methods are limited by sensitivity and resolution, making it difficult to accurately capture key intermediates. Synchrotron VUV photoionization mass spectrometry uses a high-brightness, wide-band continuously adjustable synchrotron radiation light source, combined with the molecular specificity of threshold ionization, to achieve in-situ identification and quantitative analysis of reactive intermediates in the combustion field. The photoionization efficiency curves of each mass channel can help to effectively distinguish isomers. By quantitatively measuring each intermediate and product, an accurate combustion kinetic model can be constructed. Related research can significantly enhance the depth of propellant combustion mechanism research and provide important technical support for the development of new environmentally friendly propellants and the design of combustion instability suppression strategies.

Recent publications on propellant combustion mechanism:
H. Fu, Y. Wang, B. Liu, Z. Wang*, X. Zhang, G. Liu*, Experimental and theoretical study of cyclopropanated fuel exo-Tetracyclo[3.3.1.02,4.06,8]nonane pyrolysis in a jet-stirred reactor, Fuel 361, 130702, 2024.
Q. Zhu, B. Liu, Z. Hu, S. Chen, Q. Xu, Z. Wang*, Pyrolysis and kinetic modeling study of TEMED: A potential green propellant, Proc. Combust. Inst., 40, 105426, 2024.
Q. Zhu, X. Lei, B. Liu, Z. Hu, Q. Xu, Y. Pan, B. Hou, X. Wang*, Z. Wang*, Experimental and theoretical studies of HAN and HEHN pyrolysis at low pressure, Energy Fuels 38, 6370, 2024.
K. Jin, Z. Zhi, L. Wu, Q. Xu, B. Liu, Z. Wang, Z. Tian*, Pyrolysis of norbornadiene: An experimental and kinetic modeling study, Combust. Flame 242, 112155, 2022.
Gas generation of battery thermal runaway
The research interests on gas production and energy release during battery thermal runaway mainly stem from the widespread application of lithium-ion batteries in new energy vehicles, energy storage and other fields and the accompanying safety challenges. With the increase in energy density, fire and explosion accidents caused by battery thermal runaway occur frequently, becoming a bottleneck problem restricting the development of technology. During the thermal runaway process, complex chemical processes such as electrolyte decomposition and electrode material reaction will release a large amount of heat and flammable/toxic gases (such as H2, CO, CxHy, etc.), and their dynamic generation characteristics directly affect the speed of fire spread and the level of toxic hazard. For example, under overcharge conditions, the proportion of H2 decreases and the proportion of CO increases, and the gas production rate increases significantly with the overcharge rate; the migration and erosion of reducing gases (such as C2H4 and C3H6) in the early heat accumulation stage can even accelerate the release of oxygen at the positive electrode, becoming the key trigger of the thermal runaway chain reaction. Therefore, analyzing the gas production mechanism and energy release law is of great scientific significance and engineering value for the development of thermal runaway early warning technology, optimization of battery material thermal compatibility and design of safety protection strategies.
By utilizing synchrotron radiation VUV light source and combining it with the threshold ionization technology, transient free radicals and intermediate products (such as HF, polysulfide derivatives, etc.) during the thermal runaway process can be accurately identified, avoiding data distortion caused by sampling lag or high pressure and high temperature limitations of traditional detection methods (such as gas chromatography). The application of photoionization efficiency curves can effectively distinguish isomers, and combined with in-situ analysis techniques (such as independently designed high-voltage coupling devices), the gas-producing components can be directly correlated with the decomposition reactions of internal battery materials. These technical features make SVUV-PIMS a core tool for revealing the chemical mechanism of battery thermal runaway, which not only supports the study of failure mechanisms from the material level to the system level, but also provides an experimental basis for the development of thermal runaway early warning systems based on gas signals (such as quantifying risks through TRD parameters) and new safe electrolyte systems.
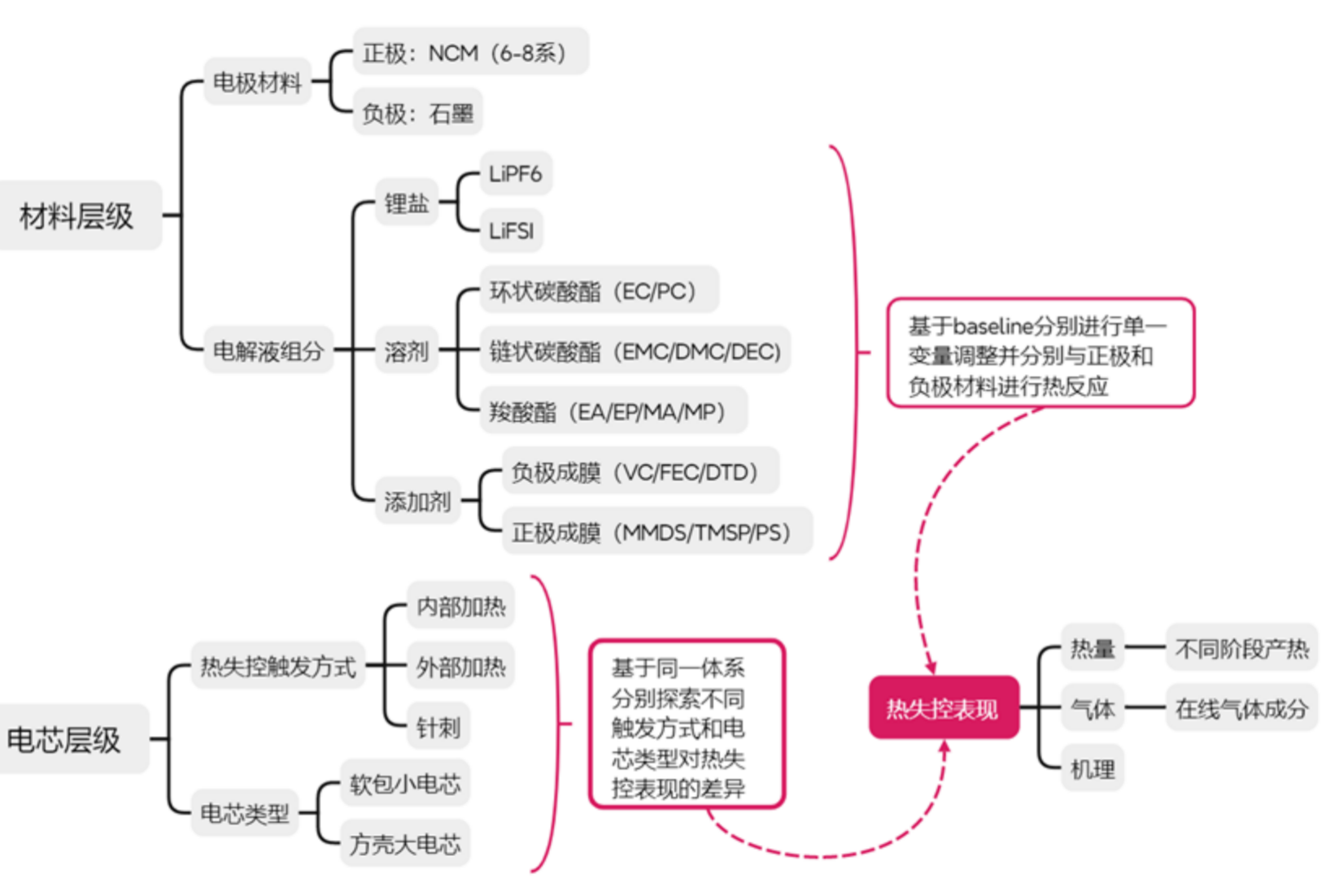
Recent publications on battery thermal runaway research:
B. Dong, L. Wang, L. Hu, J. Fang, Z. Wang*, Exploring the oxidation chemistry of diethyl carbonate in lithium-ion battery thermal runaway using SVUV-PIMS [J]. Proc. Combust. Inst, 2024, 40, 105479.
B. Dong, Y. Yu, Q. Zhu, B. Liu, K. Zhang, J. Fang*, L. Hu*, Z. Wang*, Experimental study of ethylene carbonate (EC) pyrolysis and oxidation in jet-stirred reactor by SVUV-PIMS. Combust. Flame, 2025, 274, 114002.
Yang et al., Comparison of heat and gas generation characteristics of lithium battery thermal runaway triggered by internal and external heating methods (in preparation)
Dong et al., Study on the gas generation characteristics and mechanism of electrolyte under thermal runaway conditions (in preparation)
Free radical reaction and soot formation
Persistent free radicals are a type of intermediate species that have attracted attention in many fields such as combustion, environment, atmosphere, and interstellar in recent years.
In the combustion environment, persistent free radicals are an important type of intermediate species. These free radicals obtain higher stability and longer lifespan through electron delocalization, and play an important role in the mass growth of polycyclic aromatic hydrocarbons (PAHs) and black carbon particles.
PAHs and soot aerosols can be inhaled by the human body and penetrate deep into the lungs, causing respiratory diseases and cardiovascular diseases. Soot particles can also form haze in the atmosphere, affecting air quality. In addition, soot particles are produced during the engine combustion process, forming so-called carbon deposits, which interfere with the normal combustion process of the engine, causing its efficiency to decrease, while also increasing fuel consumption. More seriously, carbon deposits may also damage key engine components, affecting their performance and life. Therefore, revealing the formation mechanism of PAHs and soot particles and understanding the basic chemical process of carbonaceous particles will help provide theoretical guidance for inhibiting the generation of these combustion pollutants from the root.
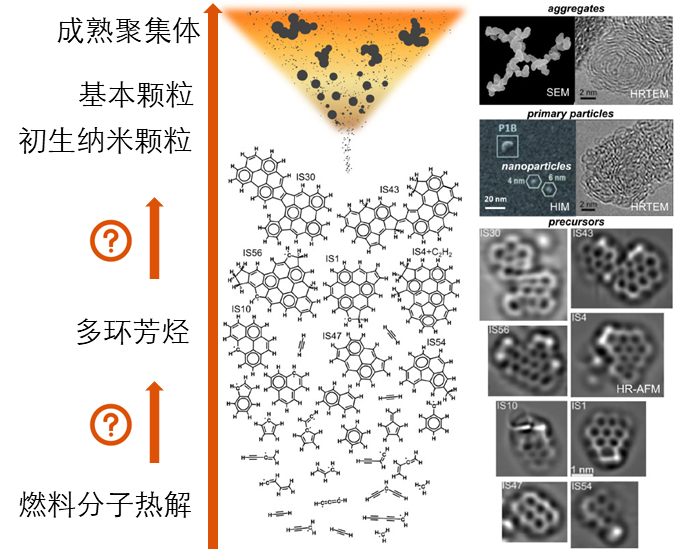
Recent publications on free radical reaction and soot formation:
H. Wang#, J. Guan#*, G. Xu, X. Mercier, J. Zhang, H. Guo, T. Yu, H. Gui, T. Huang, D. G. Truhlar*, Z. Wang*, “Resonance-Stabilized Radical Clustering Bridges the Gap Between Gaseous Precursors and Soot in the Inception Stage”, Proc. Natl. Acad. Sci. U.S.A, 2025. 122, e2503292122.
J. Zhang#, J. Gao#, H. Wang, J. Guan*, G. Xu, L. Xing, D. G Truhlar*, Z. Wang*, Less-Dominant Resonance Configuration of the Propargyl Radical Leads to a Growth Mechanism for Polycyclic Aromatic Hydrocarbons that Preserves the Cyclopenta Ring, J. Am. Chem. Soc., 147, 9283-9293, 2025.
G. Xu#, H. Wang#, J. Zhang#, J. Gao, J. Guan*, Q. Xu, D. G. Truhlar*, Z. Wang*, Combining synchrotron vacuum-ultraviolet photoionization mass spectrometry and gas chromatography-mass spectrometry for isomer-specific mechanistic analysis with application to the benzyl self-reaction, Nature Communications, 2024, 15, 10755.
H. Wang, J. Guan*, J. Gao, J. Zhang, Q. Xu, G. Xu, L. Jiang, L. Xing, D. G. Truhlar*, Z. Wang*, Direct Observation of Covalently Bound Clusters in Resonantly Stabilized Radical Reactions and Implications for Carbonaceous Particle Growth, J. Am. Chem. Soc., 2024, 146, 13571-13579.
J. Zhang#, B. Liu#, H. Wang, J. Zhang, G. Xu, J. Gao, Y. Zhao*, J. Guan*, Z. Wang, Unexpected indenyl radical formation through thermal decomposition of naphthyl radical at high temperature, Combust. Flame, 2025, 275, 114097.
Low temperature oxidation chemistry of fuels
The low-temperature oxidation chemistry of fuel plays a key role in the engine ignition process, directly affecting the knock phenomenon of gasoline engines, the combustion stability of gas turbines, and the compression ignition behavior of diesel engines. In addition, new combustion technologies that have received much attention in recent years, such as homogeneous charge compression ignition (HCCI) and controlled reactivity compression ignition (RCCI), have become research hotspots because they significantly improve combustion efficiency and significantly reduce pollutant emissions. The ignition timing control and combustion rate regulation of these new combustion technologies are highly dependent on the low-temperature oxidation chemistry of fuel. Therefore, in-depth research on the low-temperature oxidation chemistry of fuel is a requirement for traditional engines and new combustion technologies, and is of great significance for optimizing and regulating the engine ignition process.
However, the low-temperature oxidation reaction network of fuels is complex and the product types are rich, especially the measurement, qualitative and quantitative analysis of oxygen-containing intermediates represented by peroxides, which brings great challenges to low-temperature oxidation research. In response to these scientific problems, the Hefei Light Source Atomic and Molecular Physics Beamline has developed a high-quality resolution and high-sensitivity synchrotron VUV photoionization mass spectrometer. By combining it with a multi-dimensional combustion experimental platform, it has systematically carried out low-temperature oxidation chemistry research on typical engine model fuels, including detection of key low-temperature oxidation intermediates, exploration of low-temperature oxidation reaction mechanisms, construction of low-temperature oxidation kinetic models, and regulation of low-temperature oxidation reactions, providing important theoretical support for the clean and efficient combustion of engine fuels.
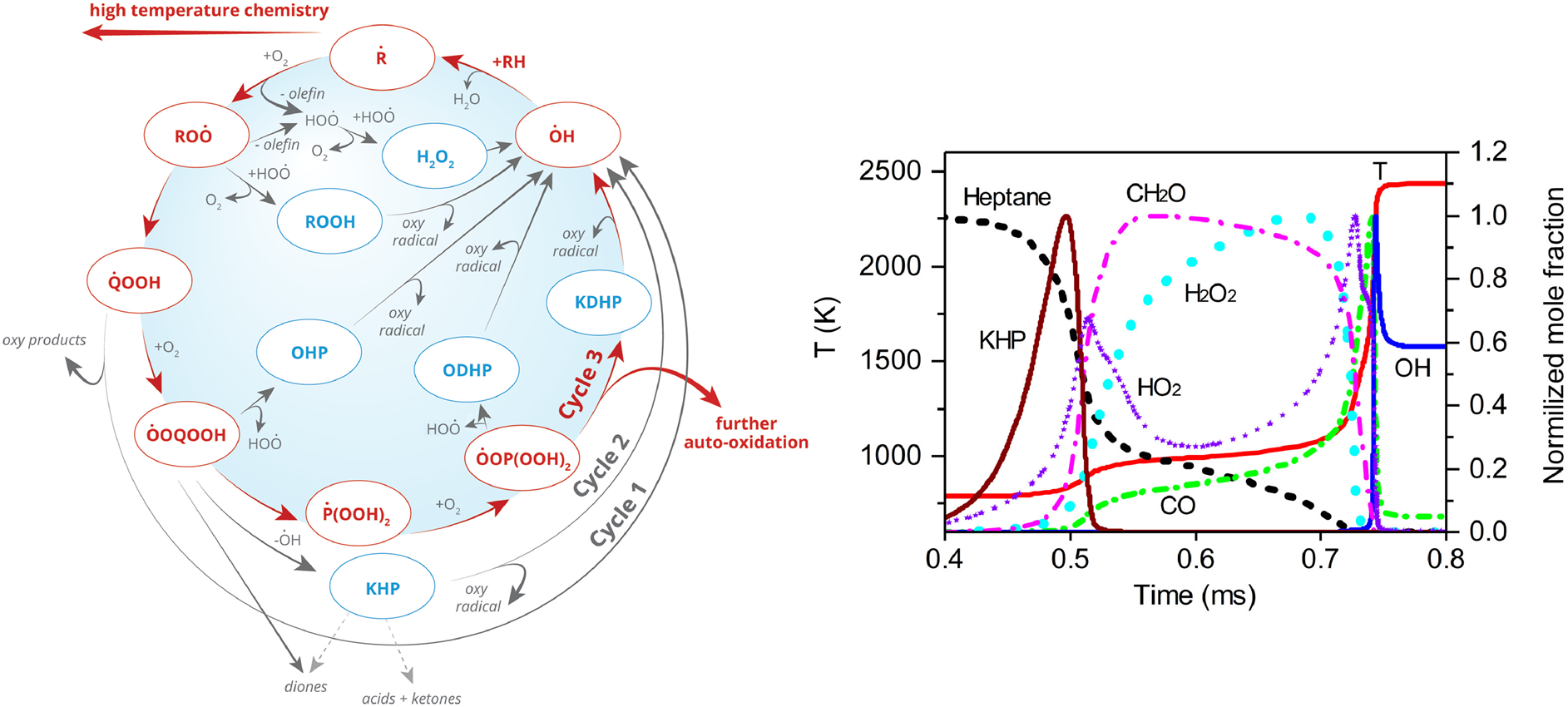
Recent publications on low-temperature oxidation chemistry of hydrocarbons
Z. Hu#, C. Xie#, S. Chen#, Q. Zhu, W. Chen, Q. Xu, B. Liu, Y. He, L. Xing*, D. G. Truhlar*, Z. Wang*, Unraveling Chain Branching in Cool Flames, J. Am. Chem. Soc., 2024, 146, 28060-28069.
L. Zhu, S. Chen, B. Liu, Q. Zhu, Q. Xu, Z. Wang*, Ozone-assisted low-temperature oxidation of acetone, Proc. Combust. Inst., 2024, 40, 105492.
Z. Hu#, Q. Di#, B. Liu#, Y. Li, Y. He, Q. Zhu, Q. Xu, P. Dagaut, N. Hansen, S.M. Sarathy, L. Xing, D.G. Truhlar*, Z. Wang*, Elucidating the photodissociation fingerprint and quantifying the determination of organic hydroperoxides in gas-phase autoxidation, Proc. Natl. Acad. Sci. U. S. A., 2023, 120, e2220131120.
W. Chen, Q. Xu, H. Lou, Q. Di, C. Xie, B. Liu, J. Yang*, H. Le Gall, L. Tran, X. Wang, Z. Xia, O. Herbinet, F. Battin-Leclerc*, Z. Wang*, Variable pressure JSR study of low temperature oxidation chemistry of n-heptane by synchrotron photoionization mass spectrometry, Combust. Flame, 2022, 240, 111946.
C. Xie, M. Lailliau, G. Issayev, Q. Xu, W. Chen, P. Dagaut, A. Farooq, S. Sarathy, L. Wei, Z. Wang*, Revisiting low temperature oxidation chemistry of n-heptane, Combust. Flame, 2022, 242, 112177.
Q. Xu, B. Liu, W. Chen, T. Yu, Z. Zhang, C. Zhang, L. Wei, Z. Wang*, Comprehensive study of the low-temperature oxidation chemistry by synchrotron photoionization mass spectrometry and gas chromatography, Combust. Flame, 2022, 236, 111797.
Catalysis and energy conversion
At present, about 85% of the world's energy supply depends on fossil fuels, and the polluting gases produced by their combustion directly aggravate environmental problems such as the greenhouse effect and frequent extreme climate. Based on this background, in-depth pollution control and energy conversion are the most effective ways to solve environmental problems. In this process, in-depth research on the microscopic reaction mechanism in the energy conversion process is of great significance for optimizing the reaction path and improving the energy conversion efficiency. However, due to their trace and transient characteristics, reaction intermediates in complex systems require advanced diagnostic technology support with high sensitivity and high temporal and spatial resolution. Synchrotron radiation light sources have unique advantages such as wide band, high brightness, high resolution and tunability, and can be combined with a variety of characterization methods for application. Among them, synchrotron VUV photoionization mass spectrometry can realize the accurate identification and quantitative analysis of reaction intermediates, free radicals and products, and is a powerful tool for clarifying the reaction mechanism of energy conversion.
The energy conversion platform based on synchrotron VUV photoionization mass spectrometry is shown below. The platform consists of three parts, including synchrotron VUV photoionization mass spectrometry, gas injection system, and flow reactor. The reaction gas is evenly mixed by the injection system and then introduced into the flow reactor. After it reacts in the reactor, the product is sampled by molecular beam and detected online by synchrotron VUV photoionization mass spectrometry. Relying on this energy conversion platform, the research team systematically carried out a series of studies covering the fields of thermal catalysis, electrocatalysis and photocatalysis, such as online detection and analysis of unstable intermediates and complex products in the reaction process, structural identification of isomers, and temperature-programmed surface reaction (TPSR) research, etc., which provides key data support for in-depth elucidation of the reaction mechanism of complex reactions.
Recent publications on catalysis and energy conversion:
L. Liu, J. Lu, Y. Yang, W. Ruettinger, X. Gao, M. Wang, H. Lou, Z. Wang, Y. Liu, X. Tao, L. Li, Y. Wang, H. Li, H. Zhou, C. Wang, Q. Luo, H. Wu, K. Zhang, J. Ma*, X. Cao*, L. Wang*, F. Xiao*. Dealuminated Beta zeolite reverses Ostwald ripening for durable copper nanoparticle catalysts. Science, 2024, 383: 94-101.
J. Ren, H. L. Li*, H. Lou, W. L. Zhou, F. Zeng, Y. Wang, X. K. Liu, C. Mebrahtu, G. Pei, J. P. Cao, T. Yao*, Z. D. Wang*, J. Zeng*. A Scenario for a Carbon-Neutral Ammonia-Fueled Engine Mediated by Catalytic NH3 Cracking and CO2 Hydrogenation. Angew. Chem. Int. Ed. 2025, 64, e202420292.
S. Xia, K. Zhao, Y. Gao, Y. Fan, C. Wang, T. Yu, H. Wang, J. Zhang, Z. Wang*, X. Zhu, Z. Zhao, A. Zheng*. Co-Feeding CO2 for Methylfuran Aromatization over Bifunctional Zeolite-Supported ZnMoO4. Angew. Chem. Int. Ed. 2025, 64, e202420779.
W. Wu, L. Luo, Z. Li, J. Luo, J. Zhao, M. Wang, X. Ma, S. Hu, Y. Chen, W. Chen, Z. Wang, C. Ma, H. Li, J. Zeng. The Importance of Sintering-Induced Grain Boundaries in Copper Catalysis to Improve Carbon-Carbon Coupling. Angew. Chem. Int. Ed. 2024, 63, e202404983.
L. Zeng, T. Yan, J. Du, C. Liu, B. Dong, B. Qian, X. Zhou, G. Su, T. Zhou, Z. Peng, Z. Wang, H. Li*, J. Zeng*. Recycling Valuable Alkylbenzenes from Polystyrene through Methanol-Assisted Depolymerization. Angew. Chem. Int. Ed. 2024, 63, e202404952.
L. Luo, T. Zhou, W. Li, H. Yan, W. Chen, Q. Xu, S. Hu, C. Ma, J. Bao, C. Pao, Z. Wang, H. Li, X. Ma*, L. Luo*, J. Zeng*. Close Intimacy between Pt-In Clusters and Zeolite Channels for Ultrastability toward Propane Dehydrogenation. Nano Lett. 2024, 24, 7236-7243.
Z. Xiong, J. Guo, Y. Deng, B. Liu, H. Lou, M. Zeng, Z. Wang, Z. Zhou, W. Yuan*, F. Qi*, Elucidating the Mechanism for Oxidative Coupling of Methane Catalyzed by La2O3: Experimental and Microkinetic Modeling Studies. ACS Catal. 2024, 14, 1267-1280.
W. Zhang*, W. Zhou, Y. Li, J. Ren, Z. Wang*. Enhanced ammonia decomposition activity over unsupported Co3O4: Unravelling the promotion effect of alkali metal. Appl. Catal. B, 2023, 330, 122644.
Back

