Study on Active Phase of Cobalt-based Catalysts in CO2 Hydrogenation
Although the chemical transformation of CO2 has been achieved in industry, the reaction requires high pressure and elevated temperature because of the chemical inertness of CO2. Up to now, the investigation of the active phase of non-noble catalysts in CO2 hydrogenation is still at the rudimentary stage.Jie Zeng's group from USTC incorporated N atoms into Co catalysts to synthesize Co4N catalysts. In CO2 hydrogenation, Co4N catalysts exhibited a turnover frequency (TOF) of 25.6 h-1 under 32 bar at 150 oC, which was 64 times as high as that of Co catalysts. The activation energy for Co4N catalysts was 43.3 kJ mol-1, less than half of that (91.4 kJ mol-1) for Co catalysts. Further mechanistic studies collaborated with the photoemission end-station at beamline BL10B in the NSRL revealed that Co4NHx was formed by adsorbing H atoms on N atoms in Co4N nanosheets in the presence of H2. The amido-hydrogen atoms in Co4NHx directly added to CO2 to form HCOO* as the intermediates. In addition, the adsorbed H2O* was found to activate the amido-hydrogen atoms via the interaction of hydrogen bonds, which facilitated the hydrogenation process. This work not only provides a way to engineer efficient, low-cost catalysts for CO2 hydrogenation via the incorporation of N atoms, but also advances our understanding of active phase of Co-based catalysts.This work has been published on Nature Energy (Nature Energy 2017, 2, 869-876.).
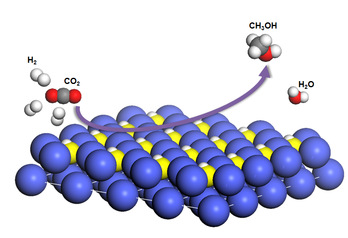
Illustration of CO2 hydrogenation over Co4N catalysts
Back

