Enhancing CO2 Electroreduction with the Metal−Oxide Interface
Prof. Xinhe Bao’s research team at Dalian Institute of Chemical Physics, Chinese Academy of Sciences designs and prepares carbon supported Au-CeOx catalyst with metal-oxide interface structure, and investigates the relationship between the Au-CeOx interface and catalytic performance of electrochemical CO2 reduction reaction (CO2RR). The CO Faradaic efficiency reaches 89.1% over Au-CeOx/C at −0.89 V vs. reversible hydrogen electrode (RHE), which is significantly higher than 59.0% and 9.8% over Au/C and CeOx/C at the same potential. The CO geometric current density over Au-CeOx/C (12.9 mA cm−2) is about 1.6 times of that over Au/C (8.3 mA cm−2) at −0.89 V vs. RHE.In situ scanning tunnelling microscopy and synchrotron-radiation photoemission spectroscopy show that the Au-CeOx interface is dominant in enhancing CO2 adsorption and activation, which can be further promoted by the presence of hydroxyl groups. Density functional theory calculations indicate that the Au-CeOx interface is the active site for CO2 activation and the reduction to CO, where the synergy between Au and CeOx promotes the stability of key carboxyl intermediate (*COOH) and thus facilitates CO2RR. Similar interface-enhanced CO2RR is further observed on Ag-CeOx, demonstrating the generality of the strategy for enhancing CO2RR.The research results provide a new way to regulate the CO2RR performance, and enrich and expand the concept of nano-confined catalysis proposed by the research team.
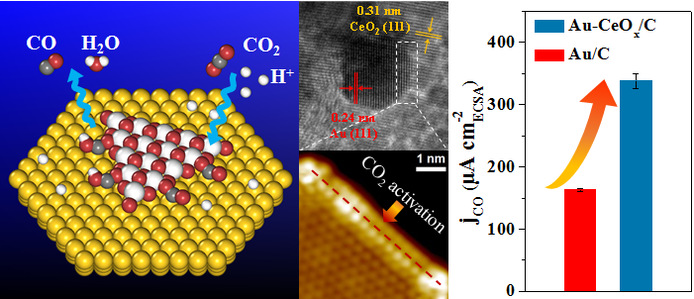
Metal-oxide interface enhances electrocatalytic reduction of CO2
Back

