Major Progress in Photocatalytic Methane-to-Ethanol Conversion by HLS Users
Methane, as the main component of natural gas and shale gas, is abundant in reserves and serves as an important carbon source for chemical synthesis. The oxidation of methane to high-value-added chemicals under mild conditions is highly promising. However, the high symmetry and stability of the methane molecule make it challenging to simultaneously achieve both high conversion efficiency and high selectivity towards a single target product, especially for those products whose formation involves C–C coupling steps. In light of this, a collaborative team including Professor Junwang Tang from Tsinghua University, Academician C. Richard A. Catlow from Cardiff University, UK, Professor Weixin Huang from the University of Science and Technology of China, and Professor Zhengxiao Guo from the University of Hong Kong published a paper titled Methane oxidation to ethanol by a molecular junction photocatalyst in Nature. For the first time, they proposed the concept of an intramolecular junction. The intramolecular junction photocatalyst CTF-1, featuring alternating benzene and triazine units, drives the coupling and oxidation of methane to ethanol with high selectivity and significantly enhanced conversion rates. The heterojunction structure not only enables efficient and long-lived charge separation after charge generation but also preferentially adsorbs H₂O and O₂ onto the triazine units and benzene units, respectively. This dual-site feature separates the site for C–C coupling (forming the ethane intermediate) from the site for generating OH radicals, thereby avoiding over-oxidation.
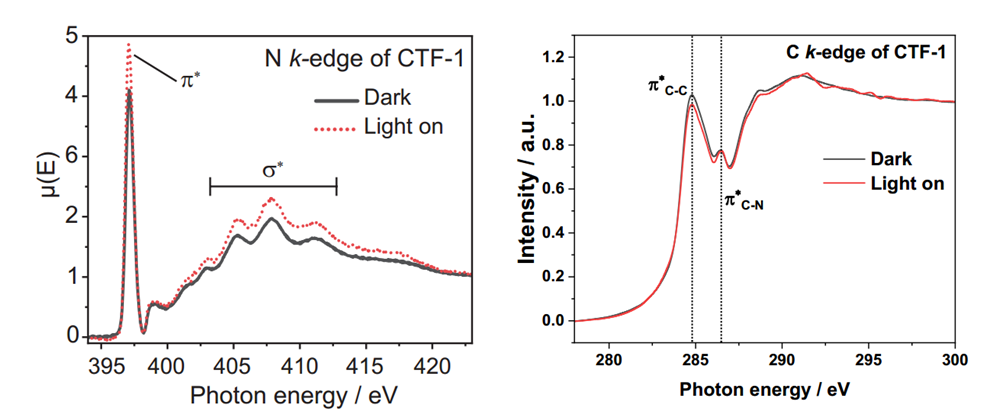
Left : N K-edge spectra of CTF-1 in dark and light condition; Right : C K-edge spectra of CTF-1 in dark and light condition.
Leveraging the in situ irradiation soft X-ray absorption spectroscopy (sXAS) capabilities of the BL10B, the team investigated the regulatory mechanism of the intramolecular junction on charge carriers. In the N K-edge spectra of CTF-1, the peak at 397.1 eV and the broad peak between 403-405 eV are attributed to π* transitions and σ* transitions of the nitrogen atoms in the triazine units, respectively. When the sample was irradiated by an LED light source, the increased intensity of the N K-edge spectra indicates that more electrons could be accepted by the N 2p orbitals, implying the accumulation of holes at the nitrogen sites within the triazine units. In the C K-edge spectra of CTF-1, the peaks at 284.8 eV and 286.5 eV correspond to C 1s → C 2p π* transitions in the benzene ring units (labeled πC-C) and the triazine units (labeled πC-N), respectively. Under LED light irradiation, the signal intensity of the πC-C peak decreased, while that of the πC-N peak remained unchanged. This suggests a reduction in the unoccupied states of the C 2p orbitals within the benzene ring units under irradiation, meaning the photoexcited electrons are likely transferred to and/or accumulated at the carbon sites of the benzene ring units.
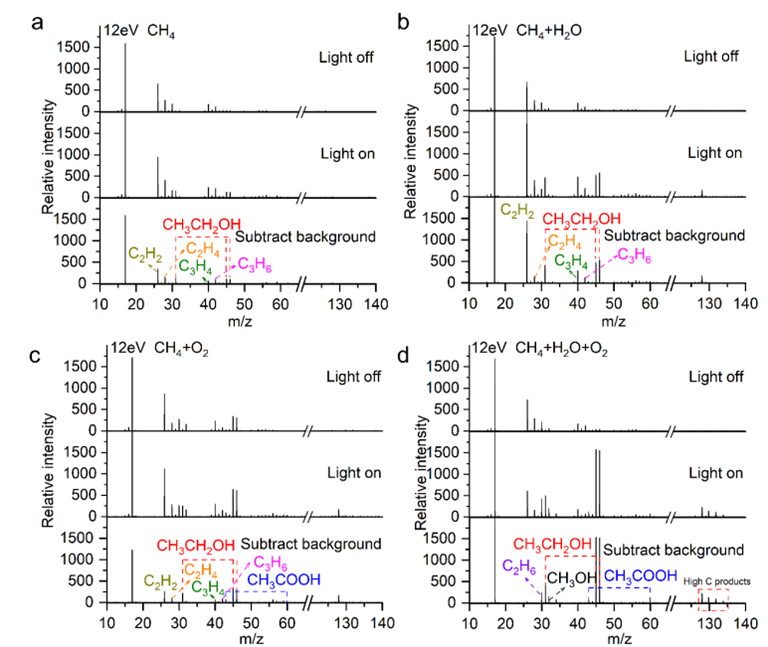
The photon energy was set to 12 eV. Mass spectra acquired in situ via synchrotron radiation photoionization mass spectrometry (SR-PIMS) for several different gas compositions flowing over CTF-1: (a) CH₄, (b) CH₄ + H₂O, (c) CH₄ + O₂, and (d) CH₄ + H₂O + O₂.
Furthermore, utilizing the Combustion and Mass Spectrometry Beamline (BL04B) at the Hefei Light Source, the team investigated the mass spectra of different gas compositions after flowing over CTF-1. When pure methane was passed through CTF-1, only C₂H₂, C₂H₄, C₃H₄, C₃H₆, and trace amounts of C₂H₅OH were generated, with the C₂H₅OH speculated to originate from residual O₂ within the system. When methane and water were passed through CTF-1, the main product species were similar, but their intensities increased, indicating that water promotes the partial oxidation of methane. When methane and oxygen were passed through CTF-1, besides the aforementioned species, low concentrations of CH₃COOH were also detected, suggesting that oxygen facilitates the formation of other products. When methane, oxygen, and water were simultaneously passed through CTF-1, the signal for the product ethanol significantly intensified, demonstrating that both oxygen and water are indispensable for the partial oxidation of methane to ethanol. Additionally, a new mass spectral peak attributable to C₂H₆ was detected at mass-to-charge ratio 30 (m/z 30), a result confirming ethane (C₂H₆) as a key reactive intermediate in the conversion of methane to ethanol.
The unique intramolecular heterojunction structure of CTF-1 enables efficient charge separation, selective water adsorption, and controllable reaction pathways, thereby suppressing over-oxidation. This intramolecular heterojunction strategy holds promise for developing highly efficient and selective catalysts for methane C–C coupling to produce C₂+ products and can be further extended to other catalytic reactions. These related findings were published in the renowned international academic journal Nature under the title Methane oxidation to ethanol by a molecular junction photocatalyst.
Paper link:https://www.nature.com/articles/s41586-025-08630-x
Back
