USTC and collaborators made a significant breakthrough in the synchrotron research on the mechanism of carbon dioxide conversion
Recently, the research team led by Professor Yao Tao from National Synchrotron Radiation Laboratory (NSRL) of University of Science and Technology of China (USTC), in collaboration with Professor Xia Baoyu from Huazhong University of Science and Technology, made significant progress in the research on carbon dioxide conversion in proton-exchange membrane (PEM) systems by comprehensively utilizing various synchrotron in situ techniques. The related research results, titled “Durable CO2 conversion in the proton-exchange membrane system”, were published in the renowned international journal Nature on February 1, 2024. (Full text link: https://doi.org/10.1038/s41586-023-06917-5)
The development of various carbon-neutral technologies is of great significance for addressing energy and environmental issues. The electro-catalytic conversion of carbon dioxide (CO2RR) based on PEM technology, which can produce high-value-added chemicals and fuels and can operate with high current and stable for a long time, is one of the promising ways to achieve industrial carbon conversion. The use of various advanced characterization techniques from the large-scale scientific facilities of synchrotron radiation holds significant scientific importance and application value. This approach is crucial for studying the structural evolution and reaction mechanism of catalysts under operating conditions and for the development of acid-stable carbon conversion catalysts and membrane electrode systems.
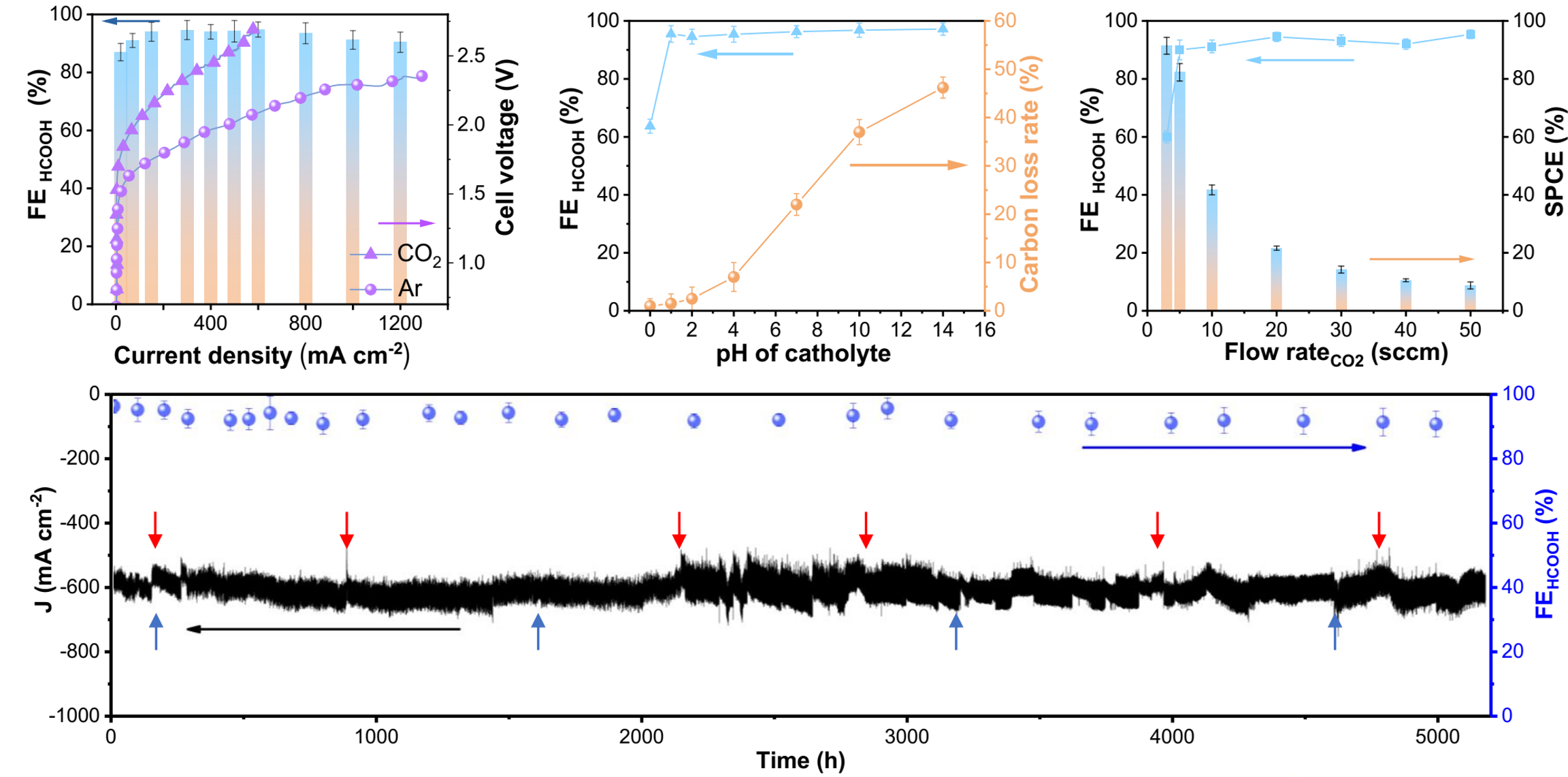
Figure 1. Activity and stability of the r-Pb catalyst membrane electrode for CO2 reduction reaction.
The researchers achieved high CO2RR activity within a wide pH range using the regenerated lead catalyst (r-Pb). Under a voltage of 2.2 V and continuous operation for 5200 hours, the faradaic efficiency of generating formic acid exceeded 93%, and the current density reached 600 mA/cm2 (Figure 1).
In order to clarify the true active structure of the r-Pb catalyst in CO2RR, researchers self-designed an operando membrane electrode CO2RR device suitable for XAFS characterization. Offline and operando characterizations were conducted respectively at the XMCD beamline (BL12B) of Hefei Light Source (HLS) and the XAFS beamline (1W1B) of Beijing Synchrotron Radiation Facility (BSRF). The operando X-ray absorption spectroscopy technique revealed that r-Pb underwent a dynamic structural evolution from Pb/PbSO4 to Pb/PbCO3 under the reduction potential of the CO2RR. The coexistence of metal state Pb and PbCO3 in a certain proportion at the reduction potential was the key factor for ultimately generating high selectivity and activity of formic acid. The researchers further conducted in-situ infrared research on CO2RR based on the in-situ infrared spectroscopy technology of beamline BL01B in HLS and the self-developed in-situ infrared device, using 13C isotopically labeled 13CO2. It was found that the gaseous CO2 on the surface of PbCO3 would first undergo a surface activation process and enter the PbCO3 lattice, and then the C in the lattice would be converted into the final HCOOH product. Combined with DFT theoretical calculations, the solid-phase dynamic transformation of the r-Pb catalyst inducing lattice carbon activation and CO2 conversion mechanism was revealed. This work provides a promising solution for efficiently converting carbon dioxide into valuable products and promoting the development of a green and sustainable society.
Professor Yao Tao from the National Synchrotron Radiation Laboratory of University of Science and Technology of China, Dr. Wang Ziyun from Auckland University of New Zealand, and Professor Xia Baoyu from Huazhong University of Science and Technology are the co-corresponding authors of this paper. This research was funded by projects from the Ministry of Science and Technology, the National Natural Science Foundation of China, etc., and received support from the synchrotron facilities of the National Synchrotron Radiation Laboratory (NSRL), Beijing Synchrotron Radiation Facility (BSRF), and Shanghai Synchrotron Radiation Facility (SSRF).

Figure 2. Schematic diagram of the proton exchange membrane CO2RR device and the characterization results of XAFS.
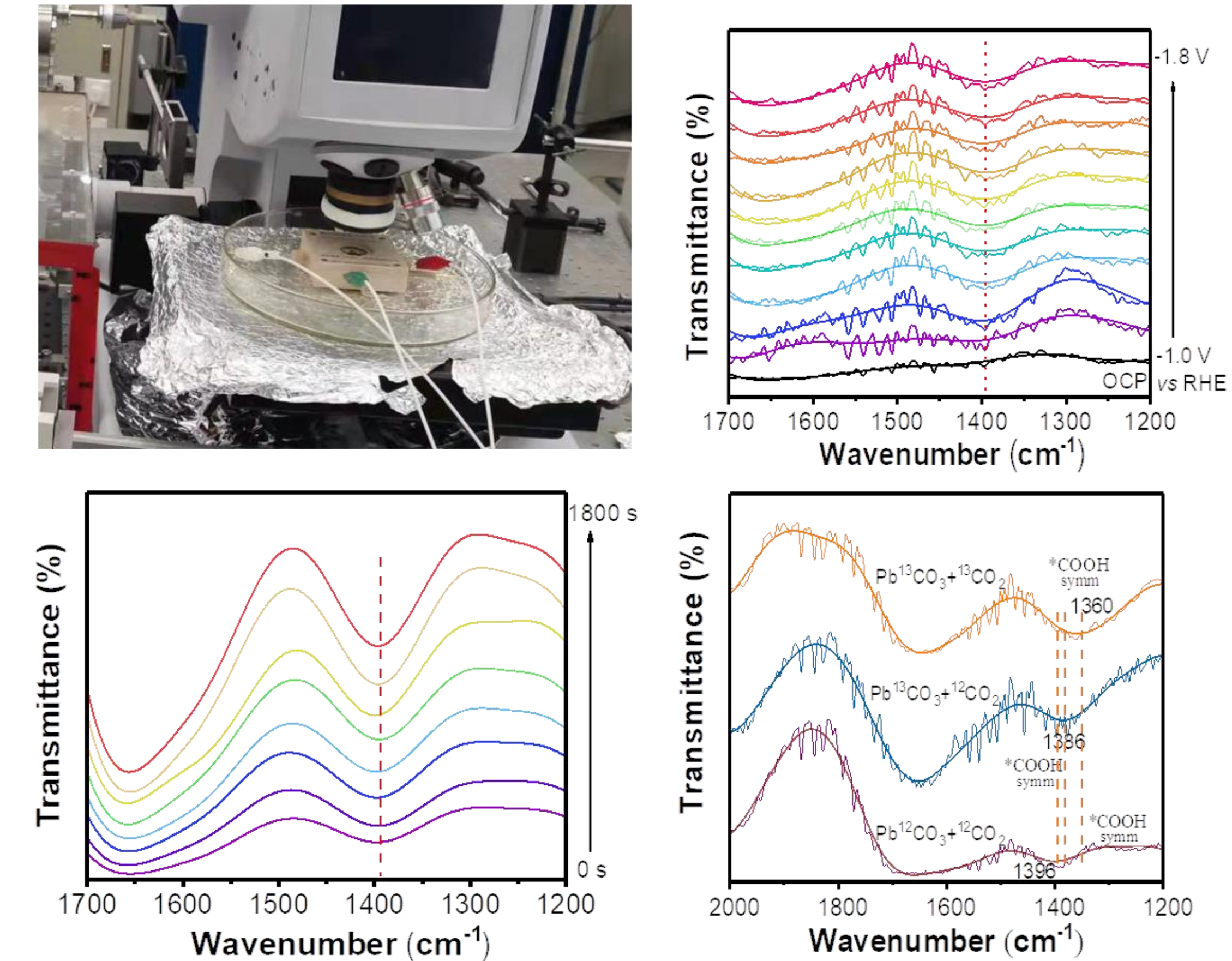
Figure 3. In-situ infrared experimental setup and results for r-Pb catalyst in CO2RR.
Back
