Useful Information
1、Project and Experiment Application
Before submitting an experimental project, please carefully review the beamline introduction and research scope, or contact the relevant beamline staff to confirm that the proposed project is suitable for this beamline. Please register and submit your project application via the Chinese Academy of Sciences Major Scientific Infrastructure Shared Service Platform. Once the project is approved and total project time is allocated, submit the experiment application on the same platform. The experimental time must not exceed the allocated total project time.
2、Lighting Plan and Operational Status
Users may apply for beamtime appropriately by consulting the lighting plan and current operational status in accordance with their experimental progress.
3、Pre-experiment Preparation
Once beamtime is confirmed, users should contact the user office staff (yhb@ustc.edu.cn, 0551-63602018) to complete radiation safety training, obtain a radiation dosimeter, user pass, and arrange accommodation.
Users are advised to carefully review the beamline introduction or user information page to familiarize themselves with available beamline equipment and experimental guidelines. For further inquiries or assistance, users should promptly contact the beamline staff to obtain the necessary information.
Before finalizing the experimental schedule, users are advised to consult with the beamline scientific staff to discuss the experiment’s feasibility, preparation requirements, and operational procedures.
Sample Preparation
Prior to conducting experiments, users should clarify sample preparation methods, sample experimental conditions, required energy levels, and experimental modes. This information can be found on the beamline website or by contacting the beamline personnel.
Cell samples: For adherent cells, it is recommended to culture them on 100-mesh nickel grids. Cells should ideally grow in the center of the grid holes, with controlled cell density to prevent cells from clustering. Approximately 10 well-dispersed cells per grid hole is optimal. Before the experiment, cells should be fixed with paraformaldehyde for about 30 minutes and then stored in PBS.
Powder samples: Prior to the experiment, dissolve the powder in ethanol or water, then deposit it onto a copper mesh. Control the sample concentration by adjusting the powder-to-solvent ratio, ensuring that the sample particles are evenly dispersed within the mesh pores. These steps should be completed before the experiment so that samples can be directly loaded at the beamline, thereby enhancing experimental efficiency.
For cell experiments, users may employ adherent cells cultured on Ni mesh or suspension cells. The following outlines the sample preparation procedure for adherent cells:
1. Determine the appropriate cell density on the Ni mesh (based on experience, approximately 7-8 cells per mesh grid is optimal).
a. Cells have nearly fully adhered to the bottom of the culture flask (i.e., ready for passaging)
b. Aspirate the culture medium, wash 2–3 times with 2 mL PBS, then aspirate the PBS
c. Digest with 1 mL trypsin (observe under an optical microscope until adherent cells become spherical and detach)
d. Add 3–4 mL culture medium to stop the digestion
e. Centrifuge at 1000 rpm for 5 minutes, then aspirate the supernatant
f. Resuspend the cell pellet in 1 mL culture medium
g. Transfer 100 μL of the cell suspension into a culture flask containing 4 mL culture medium
2. Ni Mesh Treatment
1. Polylysine Treatment:
a. Dilute polylysine to 0.1 mg/mL.
b. At room temperature, immerse the Ni mesh in polylysine for 10 to 30 minutes.
c. Rinse the Ni mesh surface with sterile water, then air dry in a laminar flow hood or dry overnight in the hood for future use.
d. Place the Ni mesh into a culture flask containing cells in the 'one-step' medium, and incubate for approximately 16 hours until cells are fully adherent (duration varies by cell type).
2. Cell Culture Medium Treatment:
a. Expose the Ni mesh to ultraviolet light for 30 minutes, then soak it in cell culture medium.
b. Place in the cell incubator for at least 2 hours, then remove and gently expel bubbles from the surface of the Ni mesh.
c. Place into a culture dish containing cells and allow the cells to adhere and grow.
4% paraformaldehyde fixation:
a. Remove all culture medium and wash 2–3 times with PBS.
b. Fix with 4% paraformaldehyde for 15 minutes, remove the paraformaldehyde, and wash 2–3 times with PBS.
c. Store the Ni mesh in PBS or freeze it directly in liquid nitrogen.
4、Research content of line stations
The soft X-ray imaging beamline primarily performs high-resolution three-dimensional structural analysis and elemental absorption edge studies, enabling acquisition of samples’ three-dimensional structures and elemental distributions. It has been widely applied in life sciences, geological environments, energy catalysis, polymer materials, and biomass conversion.
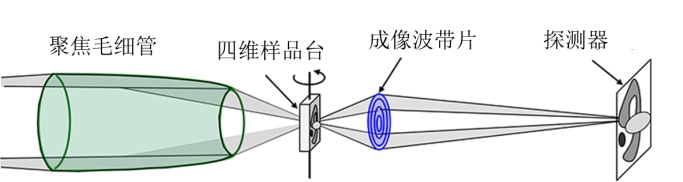
The soft X-ray imaging experimental station comprises a rotating ellipsoidal focusing system, sample stage system, imaging zone plate system, sample pre-alignment system, and CCD system. The optical layout is illustrated in the figure below.
1. Life Sciences
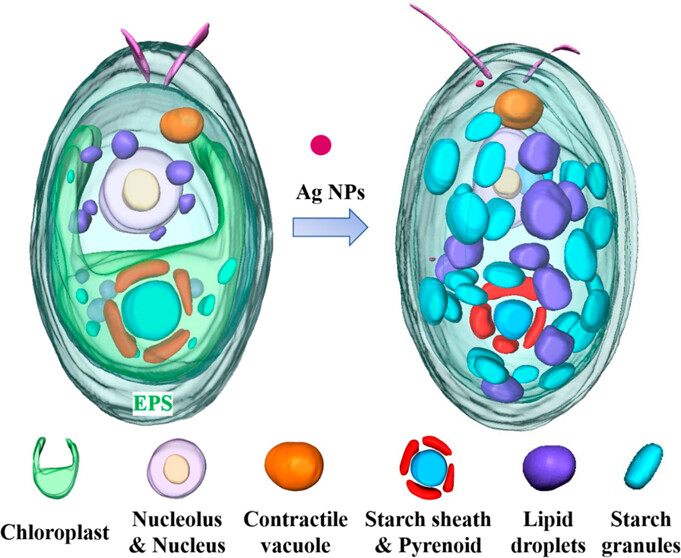
The experimental station is equipped with a liquid nitrogen cryogenic system, enabling the sample stage temperature to be reduced to 120 K. By cryogenically freezing biological samples, X-ray-induced radiation damage is minimized, allowing direct high-contrast nondestructive three-dimensional imaging of hydrated biological specimens (e.g., cells) (see figure below). This technique provides a visualization method for characterizing subcellular organelle structures, the intracellular distribution of nanomaterials, and their impact on organelles.
2. Geological Environment
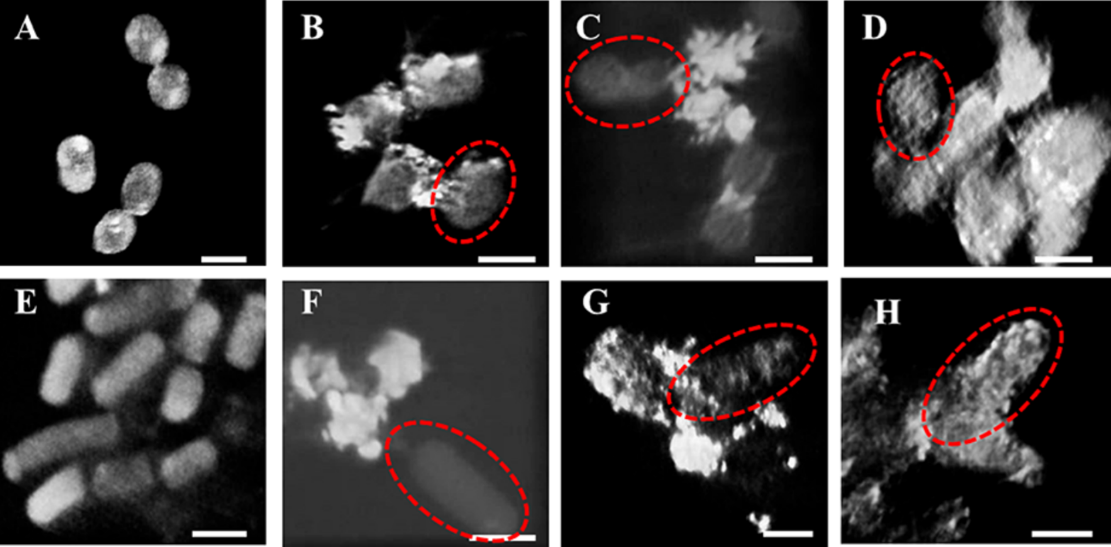
The photon energy range of the soft X-ray imaging beamline encompasses the absorption edges of nearly all critical elements found in biological systems, environmental samples (soil, minerals), and extracellular polymeric substances. This capability allows for direct visualization of fungal-mineral interface processes, offering a vital tool for investigating these interactions.
3. Energy Catalysis
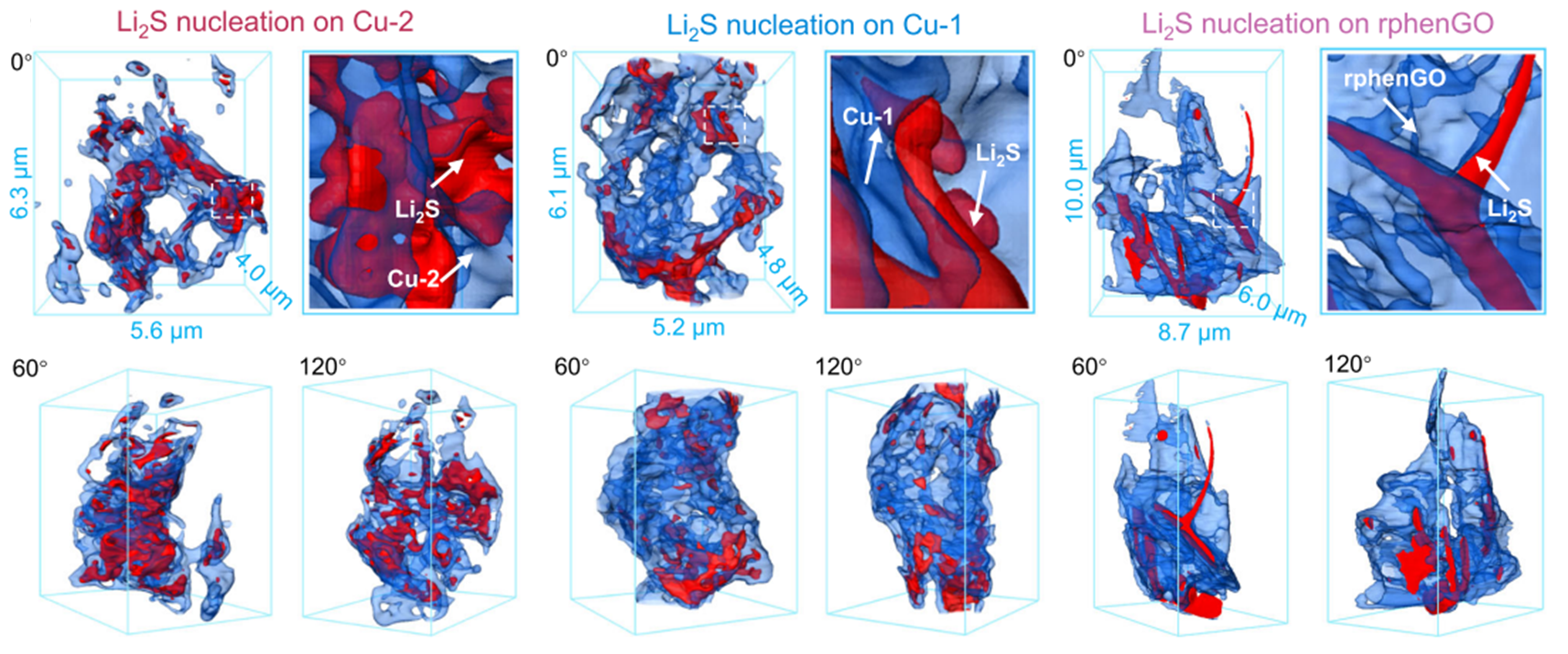
In the energy range of 260–800 keV, absorption edge imaging can be conducted for elements including C, N, O, V, Ca, Cr, Mn, Fe, and Co, enabling determination of the distribution and concentration of specific elements within samples (see figure below). This provides three-dimensional structural elemental information of key light materials relevant to energy catalysis and related industrial fields.
4. Polymer Materials
The soft X-ray imaging beamline enables three-dimensional structural and elemental imaging of polymer materials. For instance, three-dimensional imaging of carbon black-filled rubber allows clear visualization of the carbon black distribution within the rubber network. The reconstructed three-dimensional data can be used to quantitatively determine the particle size and distribution of carbon black.
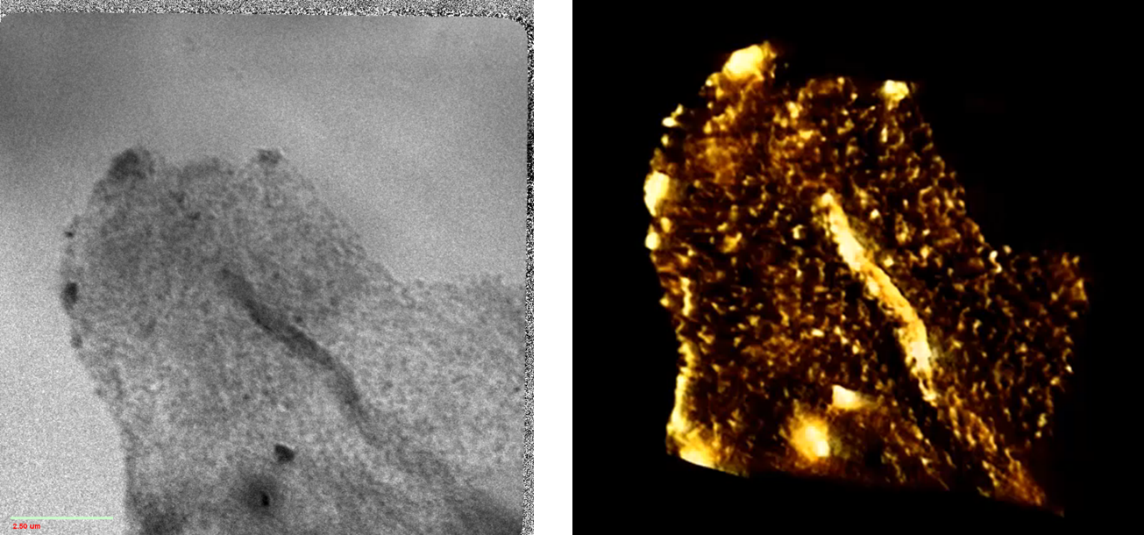
5. Biomass Conversion and Utilization
Biomass primarily consists of light elements, which exhibit strong absorption contrast in the soft X-ray range. Consequently, the soft X-ray imaging beamline can directly visualize the structure of biomass and its interactions with degradation materials, such as catalysts.
Back

