Anomalous catalytic effect of H2O in the initial reaction of Pt nanoparticle
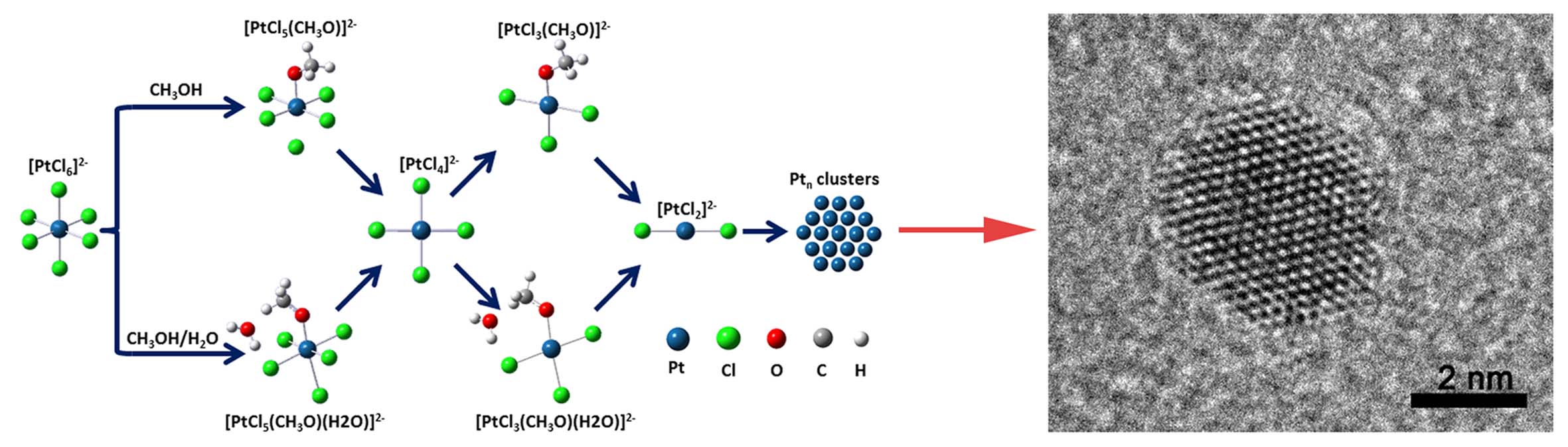
Figure: Schematic representation of the reaction pathway in both CH3OH and CH3OH/H2O solutions.
In the past few decades, noble metal nanoparticles have drawn much attention because of their promising applications in catalysis, fuel cell and gas sensing fields. Since the initial nucleation is a key step in the growth process, to understand the detailed reaction mechanism in the early stage of noble metal nanoparticles is very critical for controlling its size, morphology and properties. Song group presented a systematic study on the formation mechanism of Pt nanoparticles in methanol-water system using a combination of UV-Vis, X-ray absorption spectroscopy (XAS), liquid chromatography mass spectrometry (LCMS) and First-principle calculation methods. They demonstrated an anomalous catalytic effect of H2O on the reduction of H2PtCl6 to Pt nanoparticles. The results pointed out a transformation from [PtCl6]2- to [PtCl5(CH3O)]2- to [PtCl4]2- to [PtCl3(CH3O)]2- to [PtCl2]2- to Pt nanoparticles in a pure CH3OH solution, while a new reaction pathway proceeds from [PtCl6]2- to [PtCl5(CH3O)(H2O)]2- to [PtCl4]2- to [PtCl3(CH3O)(H2O)]2- to [PtCl2]2- to Pt nanoparticles when 10vol% water was added into CH3OH solution. It was clear indicating that the supernumerary water molecular could significantly accelerate the rate of chemical reduction and greatly shorten the reaction time, acting as a “catalyst”. This work not only elucidates the initial reaction mechanism of Pt nanoparticles, but also highlights the pronounced influence of H2O on the reaction pathway, which will provide useful insights for understanding the formation mechanism of noble metal nanoparticles and open up a high efficient way to synthesize new functional nanomaterial.
This work was supported by MOST of China, the National Natural Science Foundation of China and the Fundamental Research Funds for the Central Universities.
Source: http://pubs.acs.org/doi/abs/10.1021/acs.nanolett.5b02098
fig - 副本.jpg
Back

