Spectroscopic identification of photoactive centers in Nitrogen-doped titanium dioxide visible light photocatalysts
Metal and nonmetal doping is a popular method to modify inorganic semiconductors, which was used widely for the preparation of visible light–responsive photocatalysts from the wide band gap semiconductor TiO2. Insight into the inherent relationship between the photocatalytic function and the chemical states of dopants has been a focus of attention in photocatalysis community.
N-doped TiO2 is the most typical example of the photocatalysts. There are six kinds of chemical structures of nitrogen species in nitrogen-doped TiO2. In order to clarify the chemical state of reactive nitrogen species, the group from Fuzhou University firstly prepared the series of N-doped TiO2 materials by a post-nitridation route and then the local structure of N species doped in TiO2 was characterized in detail by XPS, EPR and near-edge X-ray absorption fine structure (NEXAFS) spectroscopes.
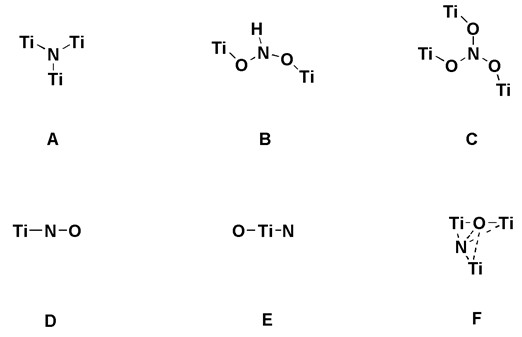
Structural modes proposed in literature
The N 1s XPS spectra of the TN-400 and TN-500 samples display only one N 1s core level peak at ca. 399.5 eV, showing that the two samples contain uniform N species (denoted as N1 species). Higher the nitridation temperature, two N 1s core level peaks are observed at 399.6 and 396.0 eV. The peak at 396.0 eV should be assignable to N3- bonded to three Ti atoms (denoted as N2 species). Based on the XPS characterization results, it can be concluded that the N1 species with a binding energy of ca. 399.6 eV are related with the visible light activity, while the N2 species with a binding energy of ca. 396 eV representing TiN species are deleterious to the visible light photocatalysis. Our EPR results indicate that the N1 species consist of the paramagnetic N1-1 species and the diamagnetic N1-2 species. For the geometric structure of the N1-1 species, there are five kinds of structures suggested in Fig.1B-F. For our samples, no H hyperfine EPR lines of NHx species were observed in ESR, so the models B can be completely ruled out. The Ti–N–O (model D) only occurs in the TN-650 and TN-700 samples, as indicated by the N 1s peak at ca. 397.0 eV and the Ti 3d peak at ca. 457.0 eV.
The geometry and bonding of N doping species were characterized by NEXAFS spectroscopy. Fig.2 shows the N K-edge NEXAFS spectra of these TN-n samples and the reference TiN. All of N-doped TiO2 samples represent two low energy features originating from transitions to the t2g (Ti 3d + N 2π) and eg (Ti 3d + N 2pσ) orbitals and higher energy features related to excitations to the N 2p and [N 2p + Ti 4sp] antibonding states. The NEXAFS results give a conclusive evidence for the direct bonding of doped N atoms with Ti in these N-doped TiO2 catalysts (excluding model C).
Therefore, it can be deduced preliminarily that the paramagnetic N1-1 species may be present in the form of the model E or F. According to the EPR result that the visible light irradiation induces the diamagnetic N1-2 species to transform into the paramagnetic N1-1 species, we proposed that the photoactive N1-2 species should be a [O–Ti4+–N3––Ti4+–Vo–] cluster containing an oxygen vacancy and a N atom. The structural model can well explain all characterization data including XPS results and the NEXAFS results. Based on the similar geometric environment of the single N atom in the photoexcited N1-2 species and the model E, one can be further ensured that the paramagnetic N1-1 species should be O–Ti4+–N2––Ti4+ (model E), instead of the interstitial N with structure of the model F.
The visible–light photoactive centers of N–doped TiO2 were identified unambiguously to be the substitutional N species with a diamagnetic [O–Ti4+–N3––Ti4+–Vo–] cluster containing an oxygen vacancy and a N atom, which acts as a source for the photogenerated electron transfer to reducible adsorbates like oxygen. After excitation by visible–light, the diamagnetic N3– species can be transformed into paramagnetic N2–species by the charge transfer, followed by the appearance of Ti3+. The photoexcited mechanism of N–doped TiO2 should be based on a surface excited state of the [Ti4+–N3–] unit. A synergistic effect of N dopant and oxygen vacancy in TiO2 contributes to the great enhancement of the visible–light photoactivity. This work was published in Journal of Catalyst (2010, 276: 201).
QQ截图20131115154837.jpg
Back

