Surface-Catalyzed C−C Covalent Coupling Strategies toward the Synthesis of Low-Dimensional Carbon-Based Nanostructures
The last decades has seen the prosperity of “top-down” techniques like lithography applied in semiconductor manufacturing industry. However, this technique will soon reach its limit due to quantum size effect, thus the future of nanotechnology should lie in the “bottom up” approach. One of the ultimate goal of the “bottom-up” nanotechnology is to build the so-called “molecular chip”, that is, all the components in the chip are based on single or several molecules, which includes molecular wires, transistors, capacitors and et al. In order to allow a considerable carrier transportation within the chip, covalent connections should be established between these components. The organic chemistry has shed light on the realization of this goal due to diverse organic reactions that can be employed for covalent linking of versatile organic building blocks. Typically, organic materials are insulators, however, the conjugated polymers (one-dimensional chains) can behave as semiconductor. Since the electronic circuits are constructed with at least two dimensions to make closed loops for the carriers. Thus, extending the polymer conjugation into two dimensions maintains huge significance in the fabrication of the “molecular chip”. Two-dimensional (2D) conjugated polymers should contain novel properties as manifested by graphene, which can be reckoned as a special 2D polymer with π-conjugation extending over the entire material.
To achieve this goal, we have employed flat substrates in the synthesis of 2D conjugated polymers to promise the planarity of them by posing a 2D confinement. The strategy for the construction of 2D polymers is the freshly developed on-surface polymerization in ultra-high vacuum (UHV), which can be easily characterized with mature surface science techniques such as scanning tunnelling microscopy (STM). Up to date, Prof. Zhu’s group had made a series of efforts to explore the polymerization of small organic haloarene molecules into large macrocycle and one- or two-dimensional (2D) networks. They have for the first time synthesized the ordered macrocycle “Hyperbenzene” molecular arrays with hexagonal lattice on a Cu(111) surface from the small haloarene 4,4’’-dibromo-m-terphenyl (DMTP) molecules. The Hyperbenzene is a large macrocycle molecules which contains 18 phenylene units. The functionalization of Cu(111) surface with these sorts of large organic molecules can hardly be realized by direct deposition because of its large weight, which may leads to its decomposition during the thermal sublimation. Except from the C-C covalently bonded quasi- zero dimensional hyperbenzene molecules, 1- or 2D phenylene chains and networks have been successfully fabricated from DMTP and TBrTP (3,5,3’’,5’’-tetrabromo-para-terphenyl) or TriBB (1,3,5-tribromo-benzene). In the fabrication of these 1- or 2D polymers, we have shed light on how to optimize the quality of these material through carefully designed precursor monomers and the selection of suitable substrates, which is the general challenge in this research field. With the experience of these works, they have also analyzed the origin of these challenges and proposed other possible promising strategies to enhance the quality of the formed 2D polymers. These efforts would contribute to the lifting of the research field to a higher stage. This work was published on Accounts Chemical Research, 2015, 48(8), 2484-2494.
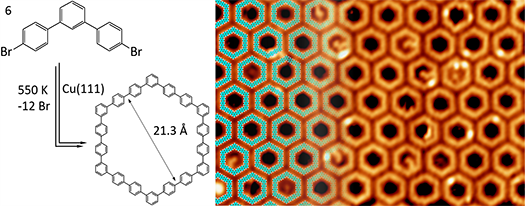
Surface-assisted synthesis of “Hyperbenzene” with Ullmann reaction
9 - 副本.png
Back

