Pillar effect on cyclability enhancement for aqueous lithium ion batteries
Pillar effect on cyclability enhancement for aqueous lithium ion batteries: a new material of β-vanadium bronze M0.33V2O5 (M = Ag, Na) nanowires
Aqueous lithium ion batteries (LIBs) can fundamentally resolve the safety problem arising from the use of highly toxic and flammable organic solvents, and effectively reduce the manufacturing cost. But many reported aqueous lithium ion battery systems have shown poor stability with capacity retention decreasing rapidly.
Prof. Yi Xie’s group in University and Science and Technology of China demonstrates the "pillar effect" (introduce appropriate structural element into the structural framework to connect the adjacent layers together by acting as "pillars") on improving cyclability of ALIBs anode materials by taking sliver vanadium oxides (SVO) system as an example. Ag2V4O11 (ε-SVO) and Ag0.33V2O5 (β-vanadium bronze) both adopt monoclinic symmetry and have similar lattice parameters, especially similar crystal structures. The key structural difference between them lies in the existence of the third vanadium site of V(3) in the structure of Ag0.33V2O5, where it also forms infinite zigzag chains along b-axis, but in five-fold square pyramidal coordination V(3)O5. Such pyramidal zigzag chains act as "pillars" to connect the [V4O11]n layers by corner-shared oxygen atoms, which changes 2D layered structure into 3D tunneled structure, thereby alleviating structural collapse and crystallinity loss during repetitive electrochemical lithiation and delithiation.
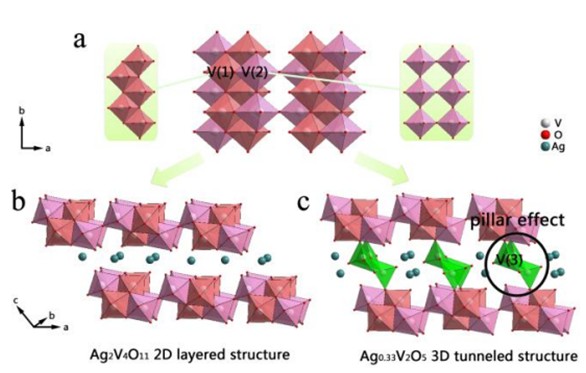
Illustration of ‘‘pillar effect’’ for V(3)O5 pyramidal zigzag chains
β-vanadium bronze Ag0.33V2O5 prepared through facile hydrothermal route has a high average V valence (4.83), which would account for larger theoretical capacity, better resistance to oxidation by air, higher electronic conductivity and electrochemical reactivity. The mixed valence state is confirmed by NEXAFS data. The two broad peaks centered around ~516 and ~523 eV can be assigned, in a first approximation, to the V L3 and L2 peaks arising from the excitations from V 2p3/2 and V 2p1/2 orbitals into empty or partially occupied V 3d states. The spin-orbit splitting between these two peaks is 6.6 eV for V2O5, 6.5 eV for AgVO3, 6.4 eV for Ag0.33V2O5, and 6.2 eV for VO2. The distinct splitting observed for β-vanadium bronze sample measured here and in analogy to measurements of bulk samples can be attributed to the presence of mixed valence of vanadium ions, providing strong evidence for charge disproportionation in its structure.
LiMn2O4 and as-prepared nanowires were chosen as cathode and anode materials, respectively, to construct batteries. LiMn2O4/Ag0.33V2O5 battery showed two discharge plateaus and delivered the first and second capacity of 103.2 and 97.2 mAh/g, respectively. 71.4% (73.7 mAh/g) and 50.0% (54.9 mAh/g) of the first discharge capacity still can be reached after 50 and 100 cycles, respectively, showing much better cyclability than LiMn2O4/Ag2V4O11 battery with only 18.7% of the first discharge capacity left after 100 cycles. Furthermore, it also showed good cyclability under different charge and discharge current density ranging from 60~120 mA/g. XRD investigation of electrode materials after long cycles confirmed the "pillar effect" arising from the pyramidal zigzag V(3)O5 chains by showing good structural stability with no degradation of the diffraction peaks. In addition, a further extension was added that, taking the advantage of this "pillar effect" into account, Na0.33V2O5 nanowires prepared through the similar route also exhibited superior cycle performance.
In this work, Xie’s group pointed out the crystallographic "pillar effect" of structural subunits by introducing effective V-O chains to transfer 2D layered structure to 3D tunneled structure, thereby to achieve large enhancement of cyclability for ALIBs. Using NEXAFS technique to verify the existence of mixed valence state of V element, their proposal was unambiguously demonstrated through the comparison of electrochemical performance of different aqueous lithium ion batteries. This work was published on J. Mater. Chem. (2011, 21: 14466).
QQ截图20131115152323.jpg
Back

