Tuning the Spin State of Cobalt in a Co-La Heterometallic Complex via Controllable Coordination Sphere of La
Paramagnetic molecular materials with their electronic structures sensitive to the environment are very attractive owing to their potential applications in molecular devices and sensors. Many research groups have made great efforts to synthesize bistable paramagnetic molecules or polymers that exist in two different spin states, which can be tuned by an external stimulus such as temperature, pressure or light. Prof. Zheng's group in Nanjing University presents a novel approach to manipulate the oxidation state of cobalt by controllable second metal coordination sphere and this work has been published on Angew. Chem. Int. Ed. (2011, 50: 5504).
Here a polytopic ligand 1,4,7-triazacyclononane-1,4,7-triyl- tris(methylenephosphonic acid) (notpH6) is utilized to generate an octahedral CoIII unit of Co(notp)3- with Ln(H2O)43+ linkages through phosphonate oxygen atoms, forming a heterometallic polymer [ls-CoIIILaIII(notp)(H2O)4]·nH2O (1a) where the CoIII ion is in a diamagnetic low spin (ls) state. The coordinated water molecules can be removed gradually on heating to 220oC, and accompanied by a change of the valence of cobalt (CoIII to CoII). It is very interesting to note that the moment of this Co-La material increases almost linearly with increasing dehydration temperature in the range of 120-220 oC. Apparently, the magnetic properties of 1a can be tuned simply by the thermal treatment at different temperatures.
Because the crystal phase of this Co-La material can be obtained before thermal treatment, the structure is readily determined by Single-crystal X-ray Diffraction. The octahedral CoIII within the structure is surrounded by three phosphonate oxygen atoms and three nitrogen atoms from the same notp6- ligand, forming a mononuclear unit of CoIII(notp)3-. This unit behaves as a quadra-dentate "ligand" and links to four LaIII ions by phosphonate oxygen atoms, leading to a two-dimensional waved layer containing 6- and 8-member rings. Each La atom is coordinated by eight oxygen atoms. Half of them are phosphonate oxygen atoms from four units of Co(notp)3- respectively, and the other are water molecules. The inter-layer space is filled with the lattice water molecules with extensive hydrogen bond interactions. After heating, the ligand field around the cobalt ion is weakened sufficiently and the equilibrium between CoIII and CoII is shifted.
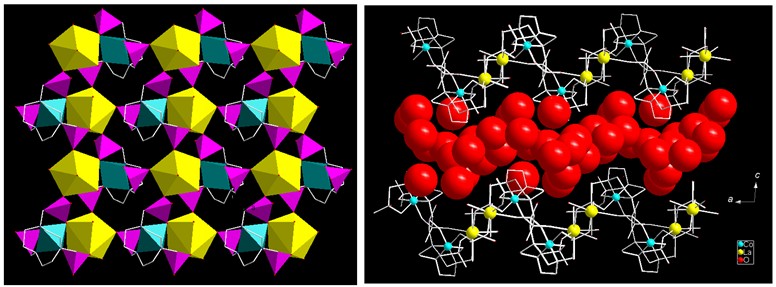
One layer and packing diagram of structure 1a along the b axis.
To investigate the change of the Co and La coordination sphere during the dehydration process, the Co K-edge and La L3-edge X-ray absorption spectroscopic measurements were conducted for samples 1a, 1a-160, 1a-180 and 1a-220. The distinct spectroscopic features of 1a-220 with respect to the other samples indicate that the local atomic arrangements around Co ions have been greatly changed. To reveal quantitatively this structural change, we performed curve-fitting for the first FT peak in the R-space using kaiser-bessel windows by taking into account the Co-O and Co-N coordination pairs. For 1a, the data fit leads to three nitrogen atoms at 1.92 Å and three oxygen atoms at 1.95 Å, which are close to those obtained by the single crystal structural determination (1.934 and 1.927 Å, respectively). And the Co-O bond length increases gradually while the Co-N bond length shrinks slightly with the dehydration temperature. Specifically, for 1a-220 a significantly elongated Co-O distance of 2.12 Å is observed, which is much longer than the Co-N bond length (1.88 Å). Furthermore, the La L3-edge EXAFS data shows that the local structure of La-O, such as the coordination number and bond lengths, remains almost the same during dehydration. Considering that around a La atom there are four coordination water molecules which would be removed upon heating to 220oC, the vacant sites of the lanthanide ion could be refilled with the neighboring oxygen atoms from the adjacent Co(notp) moieties. DFT study confirms that three O atoms from adjacent notp6- ligand chelated directly to the atom Co are able to be pulled to the lanthanide ion occupying the coordination sites released by water molecules. The coordination of these oxygen atoms to La would lead to the elongation of the corresponding Co-O distances and thus affect the electronic configuration of the Co atom. Apparently, the driving force of the geometrical rearrangement in the cobalt coordination sphere of 1a upon dehydration should be the variation around the LaIII coordination sphere.
In this work, the diamagnetic layered compound [CoIIILaIII(notp)(H2O)4]·nH2O (1a) can experience thermally induced electron transfer through gradual release of the coordination water around the La atom. Hence the magnetic properties of this compound may be modulated in a controllable manner via thermal treatment in the range 120 – 220oC.
QQ截图20131115154259.jpg
Back

