Advancement on the Oxide-supported metal model catalysts research
CeO2-supported and ZrO2-supported metal catalysts are widely used in industrial reactions. It is well known that their catalytic properties, including activity, selectivity and lifetime, which strongly depend on the interface properties referring to morphology, the valence state of metal, electronic structure and reactivity in the metal-oxide systems.
By using photoemission station in Hefei Synchrotron Radiation Facility, the researchers from National Synchrotron Radiation Laboratory, have made a series of advancements on the CeO2-supported and ZrO2-supported metal catalysts researches.
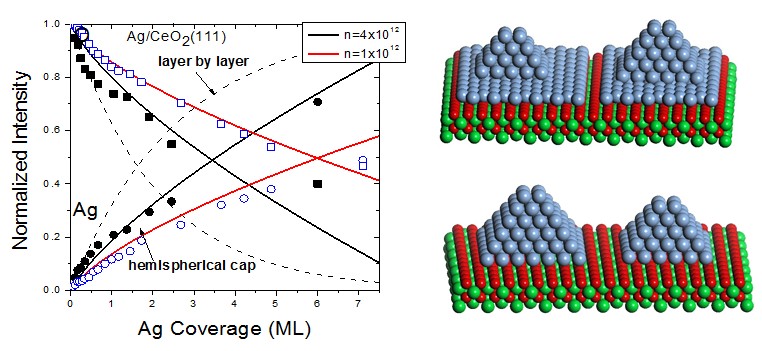 Integrated Ag 3d5/2 (circles) and O 1s (squares) XPS peak intensities, normalized to bulk Ag and the clean CeO2(111) surface, respectively, versus coverage for Ag growth on CeO2(111) films at room temperature.
Integrated Ag 3d5/2 (circles) and O 1s (squares) XPS peak intensities, normalized to bulk Ag and the clean CeO2(111) surface, respectively, versus coverage for Ag growth on CeO2(111) films at room temperature.
Synchrotron radiation photoemission spectroscopy measurements was used to investigate the growth modes, morphologies, electronic structures and thermal stabilities of metal particles supported on oxides, including Au on ZrO2 and Ag supported on CeO2. The results indicate that Ag nanoparticles initially populates defect sites on the CeO2(111) surface at room temperature, leading to a two-dimensional (2D) island growth at low coverages followed by 3D islanding at high coverages. Moreover, reduction of CeO2 upon Ag deposition was observed, which can be ascribed to the reverse spillover of oxygen atoms from the Ag-CeO2 boundary to the Ag particles. This method is valuable for the research focuses on reaction activity of Ag/CeO2 catalysts in the redox reactions. The binding energy of the Au 4f peaks shifts monotonically toward a higher binding energy with decreasing the Au particle size on ZrO2(111) surface by 0.4 eV, which is relevant to the contribution from both the initial and final state effects. Thermal annealing experiments demonstrate that Au particles are more thermally stable on the sputtered ZrO2(111) surface than on the pristine ZrO2(111) surface due to the stronger bonding of Au atoms on the surface defect sites. The results have been published on the important journal in physics chemistry science: The Journal of Physical Chemistry C (2011, 14: 6715; 2011, 21: 10744)。
QQ截图20131115164443.jpg
Back

