Arsenic Trioxide Controls the Fate of the PML-RARα Oncoprotein by Directly Binding PML
Arsenic, an ancient drug used in traditional Chinese medicine, has attracted worldwide interest because it shows substantial anticancer activity in patients with acute promyelocytic leukemia (APL). Arsenic trioxide (As2O3) exerts its therapeutic effect by promoting degradation of an oncogenic protein that drives the growth of APL cells, PML-RARα (a fusion protein containing sequences from the PML zinc finger protein and retinoic acid receptor alpha). PML and PML-RARα degradation is triggered by their SUMOylation, but the mechanism by which As2O3 induces this posttranslational modification is unclear. Here we show that arsenic binds directly to cysteine residues in zinc fingers located within the RBCC domain of PML-RARα and PML. Arsenic binding induces PML oligomerization, which increases its interaction with the small ubiquitin-like protein modifier (SUMO)–conjugating enzyme UBC9, resulting in enhanced SUMOylation and degradation. The identification of PML as a direct target of As2O3 provides new insights into the drug’s mechanism of action and its specificity for APL.
For the first time, the local structure around complex metalloprotein was analyzed using MXANS double locus method.
For the first time, the local structure around complex metalloprotein was analyzed using MXANS double locus method.
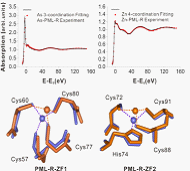
Fig . Arsenic binds to PML-R and induces a conformational change in this protein domain in vitro
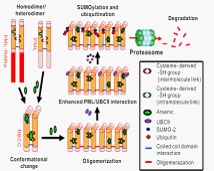
Fig . Biological consequences of the structural change in PML that is induced by arsenic binding
W020110726328061524331.png
Back

