Direct Confined-space Combustion to Monoclinic Vanadium Dioxides (VO2(M)) with Fully Reversible Metal-Insulator Phase Transition Property
Monoclinic vanadium dioxides (VO2(M)) is a prototype material for the interpreting of correlation effects in solids. Also, monoclinic VO2(M) possess a fully reversible metal-insulator phase transition between VO2(M) and VO2(R) with the benefits of huge temperature-induced resistivity changes as well as the selectively optical switches, received great interest in the industrial and scientific commands for the construction of the intelligent devices such as temperature sensors and the energy efficient smart windows.
Although VO2(R) has the superior structure stability, the synthetic strategy to VO2(M) is significantly limited during the past more than 50 years, making VO2(M) as one of the most expensive metal oxides now. And thus there presents the great demands for achieving a simple and convenient available way to realization of the formation of VO2(R). Prof. Yi Xie at Hefei National Laboratory for Physical Sciences at Microscale highlighted an available pathway to achieve monoclinic VO2(M) from direct combustion of VO(acac)2 ethanol solution in a confined space, bringing the expensive VO2(M) into the conventional laboratory synthesis era. The whole reaction process was unexpectedly convenient with short reaction time, “green” chemistry, no need of any other complex equipments and manipulations. In order to detect the exact mechanism of confined-space combustion process, the tunable synchrotron vacuum ultraviolet (VUV) photoionization mass spectrometry at National Synchrotron Radiation Laboratory was applied to perform experiments of premixed stoichiometric ethanol/oxygen flame and pyrolysis of ethanol. The photoionization mass spectra clearly give the information that the dominantly intermediate species in the ethanol flame, such as alcohol, aldehyde, H2 and CO, are reductive and inert as to the vanadium valence state. And therefore, the ethanol combustion provides the sufficient thermal energy as well as the reducing/inert atmosphere, to overcome the reaction barrier and the reducing/inert atmosphere to keep the vanadium in the +4 valence state, respectively. These two synergic effects favor the formation of thermally stable phase of V4+ oxides, and as expected the VO2(R) were formed. When the reaction temperature was decreased to room temperature, VO2(M) was obtained finally. The synthesis strategy developed here to high-quality VO2(M) then provides the new alternative possibility to settle the long-running debate over the roles played by lattice distortion or electron-electron correlations in the temperature-driven metal-insulator transition. This work has been published in Angew. Chem. Int. Ed. (2010, 49, 134) and highlighted in Nature China.
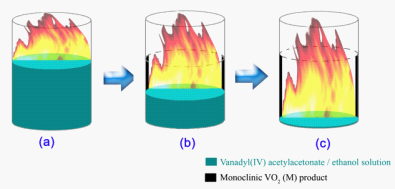
Fig. 1. Direct confined-space combustion to give monoclinic VO2(M)
图片2.jpg
Back

